2019年以来中国3D打印骨科植入物商业转化新进展(转)
阅读次数:1396 发布时间:2020-06-28
2020年6月23日,硅谷医疗3D打印创新平台3DHEALS(3dheals.com)的“专家角/ Expert’s Corner”专栏发表了由3D科学谷联合创始人朱琳撰写的专栏文章' Progress in Commercialization of 3D Printed Orthopedic Implants in China Since 2019'。本期3D科学谷将分享发表在3DHEALS的原文及中文译文。
▲3DHEALS Expert’s Corner
来源:3DHEALS
3DHEALS
Expert’s Corner
In our previous expert corner article published last year, ‘3D Printed Orthopedic Implants in China and the Challenges in Commercialization’, we reviewed the commercialization profiles of orthopedic 3D printing implants in China and showed that the Chinese orthopedic medical device manufacturing market has made slow progress towards commercialization compared to Europe and the United States.
However, since then, there was a clear acceleration of commercial transformation in China’s orthopedic 3D printing implant industry. In this article, we review the current commercialization progress of 3D printed implants in China involving: A) new 3D printed implant products; B) customized medical device supervision and management regulations; C) product registration technical review guidelines; D) group standards.
A. Newly listed 3D printing implant products
AK Medical

▲Figure 1: AK Medical 3D printed implants
Source: AK Medical
AK Medical is a pioneer in the research, development, manufacturing and commercial application of 3D printing metal implants in China. It is also a standardized orthopedic 3D printing metal implant manufacturer in the Asia-Pacific region.
In 2015-2016, AK Medical received three registration certificates from the China’s National Medical Products Administration (NMPA) for its 3D printed implants, including acetabular components, vertebral prostheses, and intervertebral fusion. In 2020, AK Medical received two more 3D printing implant products cleared by the NMPA. The newly cleared products are metal 3D printed pelvic defect matching prosthesis and metal 3D printed customized cervical fusion.
3D printing implants have become one of AK Medical’s core products. In 2019, the revenue of these products exceeded RMB 123 million, accounting for 13.3% of the total revenue.
JUST Medical

▲Figure 2:3D printed trabecular bone hip cup-standard
Source: JUST Medical
On July 12, 2019, Just Medical’s “trabecular bone hip prosthesis” was cleared by NMPA. JUST Medical has developed 3 types of 3D printed hip cups, namely trabecular bone hip cup-DDH, trabecular bone hip cup-standard, and trabecular bone hip cup-revision. All three models are manufactured through Electron Beam Melting (EBM) process.
B. Customized Medical Device Supervision and Management Regulations
On January 1, 2020, China’s “Regulations on the Supervision and Administration of Personalized Medical Devices (Trial)” was officially implemented. The interpretation of the regulations by NMPA includes four key points:
1. Customized implants are applicable, but patient-matched (or patient-specific) implants are not applicable. Personalized medical devices refer to medical devices designed and manufactured by medical device manufacturers according to the clinical needs of authorized medical personnel in medical institutions to meet the personalized requirements of patients. They are divided into customized medical devices and patient-matched medical devices.
2. Supervise by putting on record
Unlike standardized mass-produced medical devices, customized medical devices do not need to go through a long period of experimentation and approval process. However, there is requirement to implement pre-market documentation according to regulations.
Customized medical device manufacturing enterprises and medical institutions jointly act as documenting parties. Before producing and using customized medical devices, they should report to the drug regulatory department of the province, autonomous region, or municipality directly under the Central Government where the medical device manufacturing enterprise is located (the imported product is where the agent is located). From the perspective of risk control, customized medical devices cannot be entrusted to mass production, and the recording party should have the corresponding conditions.
3. Special requirements for design and processing involve personnel management, design and development, quality control, and traceability management.
4. When the use of a customized implant meets the pre-market approval requirements, such implants can be declared for registration.
According to the market observations of 3D Science Valley, after the formal implementation of the regulations by the NMPA, AK Medical and CHUNLI have submitted a number of applications for the record of the customized 3D printed implants.
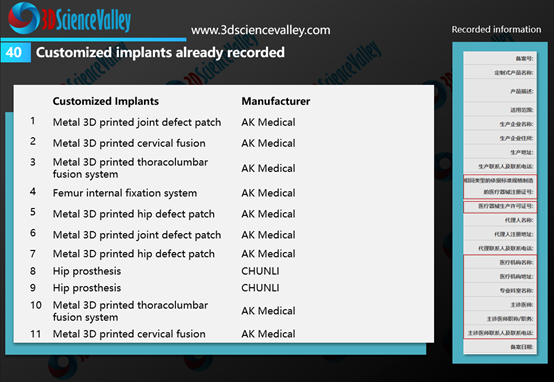
▲Figure 3: Customized implants already recorded by AK Medical and CHUNLI
Source: 3D Science Valley ” White Paper of 3D Printing Orthopedic Implant 3.0″
As can be seen on the right side in Figure 3, the filing information needs to indicate the hospital and doctor who used the 3D printed implant, that is, this implant is limited to the doctor mentioned in the filing, and cannot be used for other purposes.
C. Guidelines for Technical Review of Product Registration
From the global perspective, the rapid commercialization progress was observed for 3D printed acetabular cups and spinal implants (especially fusion cages). The figure below shows the timeline of these 3D printed implants from its earliest commercialization in 2007 and onwards.
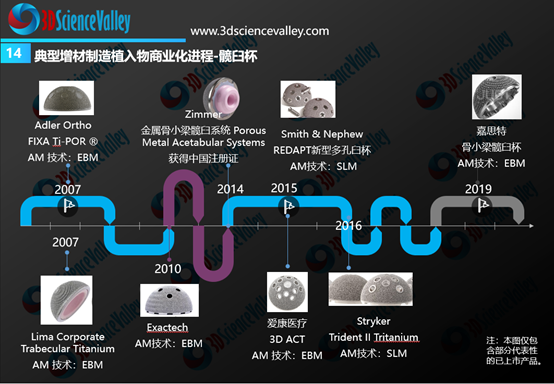
▲Figure 4: 3D printed hip cup commercialization progress
Source: 3D Science Valley ” White Paper of 3D Printing Orthopedic Implant 3.0″
The China NMPA is also promoting the commercial transformation of 3D printed hip cups, spinal fusion cages, and artificial vertebral implants. In 2019, it released a draft for consultation on the technical review guidelines for the above three types of standardized 3D printed implants.
D. Group standards
Promoted by the 3D Printing Medical Devices Committee of China Association for medical devices industry (3D CAMDI) the first five group standards for 3D printing medical devices in China were officially implemented on July 20, 2019 include:
1. Mechanical Equivalent Model of Customized Medical Device.
This standard specifies the methods for obtaining and analyzing mechanical equivalent models of customized medical devices. This standard applies to the mechanical analysis of some or all additive manufacturing products.
This standard specifies the requirements and precautions for the development of mechanical models to predict the loading of mechanical equivalent models in the evaluation of customized medical device mechanical equivalent models. The methods in this standard can be used to evaluate the equivalent mechanical models of a series of customized medical devices to meet the requirements of the devices. The procedures for model verification and validation are recommended in this standard to help determine whether the analysis of the mechanical equivalent model complies with the recommended guidelines. This standard recommends what should be included in the analysis report.
2. Special Requirements for Quality Control System of Customized Medical Devices
Customized medical devices have an important role in promoting the development of personalized medicine. However, due to the individuality of the design and production of such products, it is difficult for conventional testing and evaluation methods to ensure the safety and effectiveness of such products. Therefore, the quality control process of such products, especially high-risk customized medical devices, are particularly important. This standard specifies the special requirements for customized medical device quality control systems.
This standard applies to customized medical devices, which include additive manufactured implantable and non-implantable medical devices. This standard is a special requirement for the quality management specification of the entire manufacturing processes of customized medical devices.
These system requirements and process control minimize the risk, guarantee traceability of products, unify the mode of medical and industrial interactions, and strengthen the verification and confirmation of the design. The processing technology parameters should be met to ensure product quality.
Customized medical device design and production enterprises need medical device registration certificates and medical device production licenses, and cannot expand activities to other medical device registration certificate holders, and are not allowed to outsource production.
3. Matched Artificial Temporom and ibular Joint
This standard specifies the product design and type, material, design evaluation, test method, quality control, manufacturing, sterilization, packaging, and information provided by the manufacturer of matched artificial temporomandibular joints.
This standard is applicable to matching artificial temporomandibular joints. This product is suitable for the repair or reconstruction of temporomandibular joints and adjacent bone tissues in oral and maxillofacial surgery, head, neck, and nose surgery.
4. Internet of customized Additive Manufacturing medical devices and general requirements for realization conditions
This standard specifies the terms and definitions for the implementation process of customized additive manufacturing (3D printing) medical device products, related parties, related responsibilities, Internet information platform implementation conditions, development and maintenance, and security requirements under the Internet conditions.
5. Monitoring and judging indicators and receiving conditions of the interactive process of Customized medical devices for Doctor and Engineer.
This standard applies to customized medical devices such as bones, joints, and oral hard tissue. Clarify the process and method of customized medical device quality assurance, standardize the relevant behaviors of medical-industrial interaction of customized medical devices, and realize the sharing and communication of medical-industrial information, monitoring and data acquisition, design and development, and devices from clinical needs to production to clinical use. The whole process of production, delivery, acceptance and follow-up.
Conclusion:
The lack of relevant regulations and standards is the main challenge restricting the industrial application of 3D printed medical devices in China. Since 2019, in addition to the newly-listed products, China has made breakthrough progress in 3D printing implant-related technical review guidelines, management regulations and group standards. The establishment and improvement of laws and standards will promote China’s 3D printed orthopedic implants to enter the road of accelerated industrialization.
在2019年“3DHEALS Expert’s Corner”专栏文章《3D打印骨科植入物在中国的应用概况以及商业转化中的挑战》中,我们回顾了2019年之前,中国的骨科3D打印植入物商业转化概况。相比欧美市场,中国骨科医疗器械制造商在3D打印骨科植入物商业转化方面的进展较慢。
不过,2019年以来,中国骨科3D打印植入物的商业转化出现了明显的加速跑趋势。在本期专栏中,我们将从中国2019年以来新增上市3D打印植入物产品、定制式医疗器械监督管理规定、产品注册技术审查指导原则、团体标准四个角度,回顾2019年至今,中国骨科3D打印植入物的商业化进展。
l 新增上市3D打印产品
爱康医疗
爱康医疗是中国3D打印金属植入物研发、制造与商业应用等方面的开拓者,同时也是亚太地区规范化的骨科3D打印金属植入物制造商。
2015-2016年,北京爱康宜诚医疗器材股份有限公司从国家药品监督管理局获得了三个3D打印植入物注册证:髋臼部件和椎体假体、椎间融合器。2020年,爱康新增两款获得国家医疗器械监督局Ⅲ类器械注册证的3D打印植入物产品,分别是:金属3D打印骨盆缺损匹配假体与金属3D打印定制化颈椎融合体。
3D打印植入物已成为爱康医疗的核心产品之一。2019年3D打印产品营收超过1.23亿元,占总收入的13.3%。
嘉思特医疗
2019年7月12日,嘉思特的骨小梁髋关节假体产品获得国家医疗器械监督局Ⅲ类器械注册证。嘉思特开发了3种型号的3D打印髋臼杯:骨小梁髋臼-DDH与骨小梁髋臼-标准型与骨小梁髋臼-翻修。3种型号均采用电子束熔融3D打印技术制造。
l 定制式医疗器械监督管理规定
2020年1月1日中国《定制式医疗器械监督管理规定(试行)》正式实施。中国国家药品监督管理局对该规定的解读,包括四个关键要点:
1. 定制式植入物使用,而患者匹配型不适用;
个性化医疗器械是指医疗器械生产企业根据医疗机构经授权的医务人员提出的临床需求设计和制造的、满足患者个性化要求的医疗器械,分为定制式医疗器械和患者匹配医疗器械。
2. 采用备案的方式进行监管;
与标准化批量生产的医疗器械不同,定制式医疗器械无需经过漫长的实验与审批管理周期,而是按规定实行上市前备案管理。
定制式医疗器械生产企业与医疗机构共同作为备案人,在生产、使用定制式医疗器械前应当向医疗器械生产企业所在地(进口产品为代理人所在地)省、自治区、直辖市药品监督管理部门备案。从风险控制的角度出发,定制式医疗器械不得委托生产,备案人应当具备相应条件。
3. 设计加工特殊要求涉及人员、设计开发、质量控制、追溯管理。
4. 当一种定制式植入物的使用数量达到上市前审批要求之后,可对此类植入物注册。
根据3D科学谷的市场观察,在该规定正式实施后,爱康医疗与春立正达开展了多个定制式植入物的备案申请。

▲图:爱康与春立正达已备案的定制式植入物
来源:《3D打印与骨科植入物白皮书3.0》(七月发布)
从上图中的右图可以看到,备案信息中需要注明使用植入物的医院、医生,也就是这款植入物仅限于备案中提及的医生使用,而不能在其他用途中使用。
l 产品注册技术审查指导原则
从全球骨科3D打印植入物商业转化情况来看,进展最快的是3D打印髋臼杯与脊柱植入物(尤其是融合器)。下图显示了全球范围内典型的3D打印髋臼杯产品,3D打印髋臼杯的商业化最早在2007年就开始了。
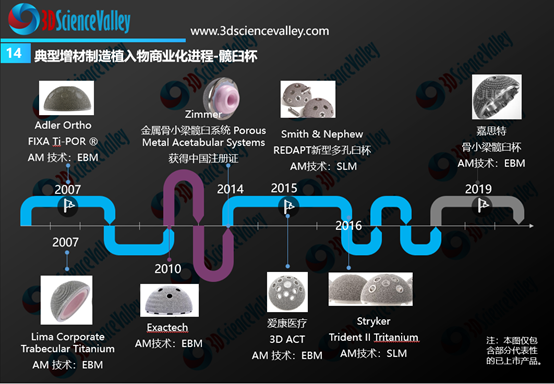
▲图:3D打印髋臼杯商业化进展
来源:《3D打印与骨科植入物白皮书3.0》(七月发布)
中国国家医疗器械监督管理局也在推动髋臼杯、脊柱融合器与人工椎体3D打印植入物的商业转化。2019年,他们发布了以上三类标准化3D打印植入物的技术审查指导原则征求意见稿。
l 团体标准
在3D打印医疗器械专业委员会的推动下,中国3D打印医疗器械第一批五项团体标准在2019年7月20日正式实施。
第一批五项团体标准包括:《定制式医疗器械力学等效模型》、《定制式医疗器械质量体系特殊要求》、《匹配式人工颞下颌关节》、《定制式增材制造医疗器械的互联网实现条件的通用要求》、《定制式医疗器械医工交互全过程监控及判定指标与接收条件》。
l 总结
相关法规和标准的缺失,是制约3D打印医疗器械在中国产业化应用的主要挑战。2019年以来,除了新增的上市产品,中国在3D打印植入物相关技术审查指导原则、管理规定以及团体标准方面取得了突破性的进展。法规与标准的建立、完善,将推动中国3D打印骨科植入物进入产业化加速跑之路。
本文转自3D科学谷





